Binding Kinetics
Binding kinetics of test compounds is often measured in competition binding assays, using the Motulsky and Mahan equation to quantify the binding rates. We have extended this equation to handle some new scenarios. First, to handle rapidly-dissociating compounds. Second, to handle new ways of setting up the assay, so you can do the experiment the way you want.
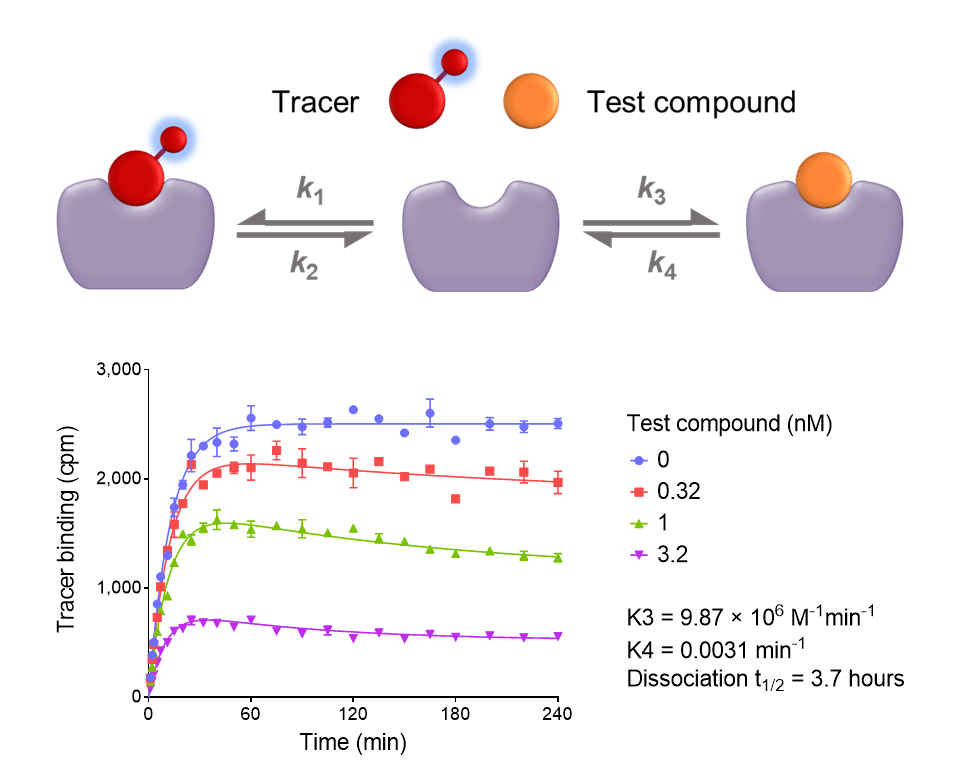
Binding kinetics is typically measured using the Motulsky and Mahan equation. We have extended this equation to handle some new scenarios. Check out the PowerPoints or visit the GraphPad curve-fitting guide here.
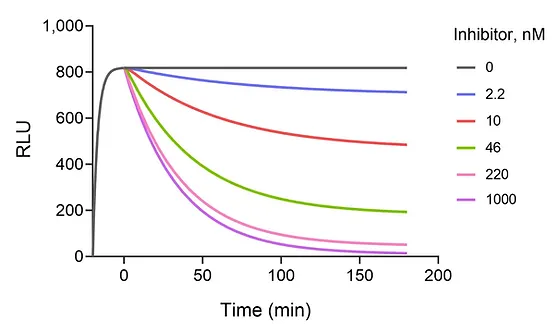
First, to handle rapidly-dissociating compounds, which give false fits to the original equation.
Second, to handle new ways of setting up the assay, so you can do the experiment the way you want.
Related
Resources
Prism technical note
Extensions of the kinetics of competitive binding equation for rapid competitor kinetics or alternative assay designs
Rapid dissociation
How to analyze competition kinetics when the competitor dissociates rapidly
New Assay Formats
How to analyze competition kinetics with alternative assay designs

Why Choose Pharmechanics?
Pharmechanics was founded by Dr Sam Hoare in 2017, with the goal of helping pharmacologists analyze, interpret and apply their data to accelerate drug discovery and basic research on drug targets. See testimonials here to see how I can support your science.
Sam is a globally-recognized expert on industrial, applied and theoretical pharmacology. As Lead Pharmacologist at Neurocrine Biosciences, his leadership, experience and insights were instrumental in the pharmacological optimization of three novel FDA-approved therapeutics, and several more compounds in clinical development.